Medical Device CDMO
Smartvein Medical Technology can provide customers with a ful ange of services from product development to sample proofing, verifica-tion, product registration to mass production.
In the ever-evolving landscape of healthcare, the demand for cutting-edge medical technology is at an all-time high. As the industry continues to advance, the need for reliable, efficient, and innovative solutions has never been more critical. Enter Smartvein Medical Technology, a pioneering company dedicated to transforming the way medical devices are developed, verified, and brought to market. With a comprehensive suite of services that spans the entire product lifecycle, Smartvein is your trusted partner in navigating the complexities of medical technology.
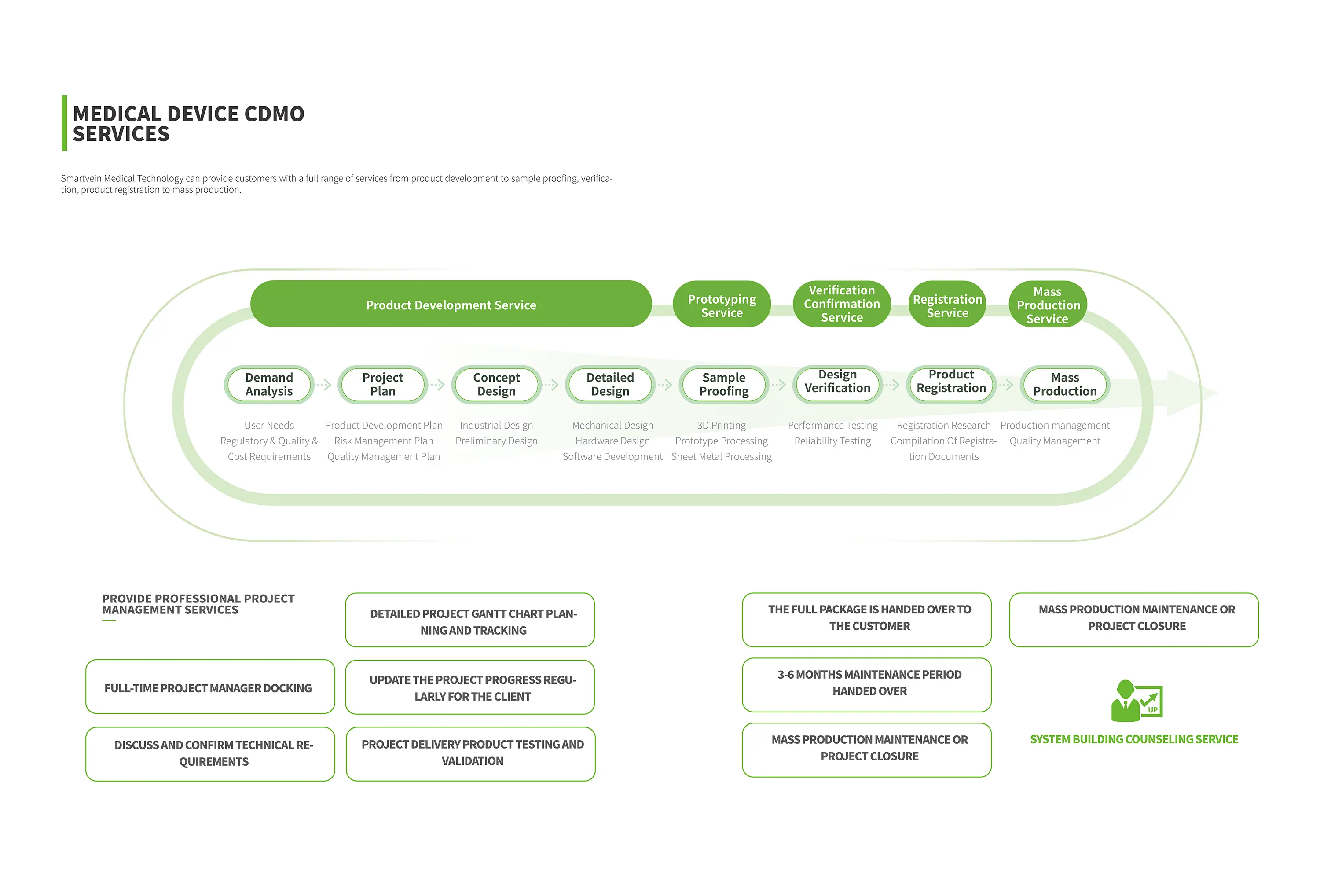
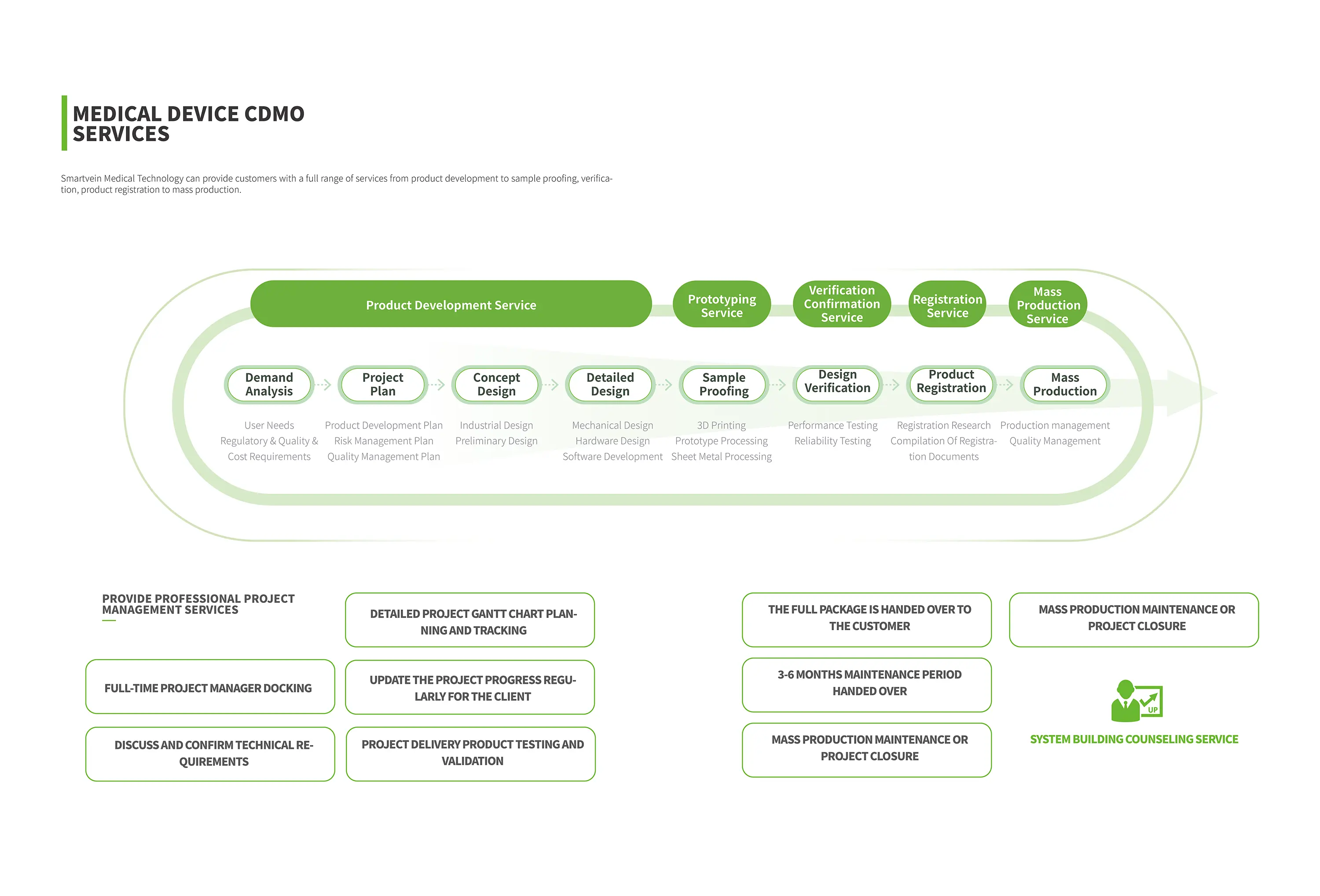
Product development service
Sample proofing service
Validation service
Registration service
Mass production service
01 Requirement Analysis
User needs, regulatory needs
Quality requirements, cost requirements
02 Project plan
Product development plan
Risk management plan
Quality control plan
03 Conceptual design
lndustrial design
Preliminary design scheme
04 Detailed Design
Structural design
Hardware design
Software design
05 Sample making
3D printing
Hand plate processing
Sheet metal working
06 Design verification
Performance test
Security testing
07 Product registration
Registration research
Registration strategy planning
Registration document writing
08 Mass production
Production management
Quality control
PROVIDE PROFESSIONAL PROJECTMANAGEMENT SERVICES
Detailed project Gantt chart planning and tracking
Full-time project manager docking
Update the project progress regularly for the client
Discuss and confirm technical requirements
Project delivery product testing and validation
System building counseling service
The full package is handed overto the customer
Mass production maintenance or project closure
3-6 months maintenance period handed over
Mass production maintenance or project closure
Medical Device CDMO: A Comprehensive Guide from Concept to Commercialization
1. Definition and Value of Medical Device CDMO
AMedical Device CDMO (Contract Development and Manufacturing Organization) is a third-party service provider specializing in end-to-end development and scalable production for medical device companies. Its core value lies in integrating R&D resources and manufacturing expertise to reduce technical barriers and financial burdens, accelerating product time-to-market. The CDMO model spans the entire lifecycle—fromconcept validation, prototyping, clinical trial support, tofinal production—and is particularly tailored for innovative, complex Class II/III medical devices.
2. Service Principles and Technical Support
CDMOs operate onmodular workflows anddigital collaboration platforms, leveraging cutting-edge technologies for precision manufacturing. Key tools include:
•Computer-Aided Design (CAD) andFinite Element Analysis (FEA) for optimizing device performance.
•3D printing for rapid prototyping and iterative design.
•Automated production lines ensuring batch-to-batch consistency.
Critical processes likebiocompatibility testing, sterilization validation, andquality management systems adhere to global standards such asISO 13485 andFDA 21 CFR Part 820.
3. Typical Applications and Product Types
CDMO services are widely adopted in fields like:
•Orthopedic implants, cardiovascular devices, minimally invasive surgical tools, andintelligent wearables.
Specialized expertise shines in:
• Complex structures (e.g., artificial joints).
• High-precision micro/nano devices (e.g., drug-eluting stents).
• Sterilization-sensitive products (e.g., heart valves).
For startups or teams lacking production capabilities, CDMOs offerend-to-end solutions to bypass costly self-built facilities.
4. Competitive Advantages Over Traditional Contract Manufacturing
CDMOs differentiate through:
1.Technical Depth: Cross-disciplinary teams skilled in materials science, biomechanics, and clinical translation.
2.Flexible Capacity: Agile production lines accommodating small-batch orders and rapid scaling.
3.Regulatory Agility: Proactive adaptation to global regulatory changes (e.g., EU MDR, FDA updates).
Additional strengths includeopen innovation labs for joint R&D, shortening development cycles.
5. Key Design Considerations for CDMO Alignment
Designers must prioritize:
•Regulatory Readiness: EmbedDesign for Manufacturability (DFM) early to align with target markets (e.g., FDA, CE).
•Modular Architecture: Standardized interfaces and modular components for production flexibility.
•Material Selection: Prioritize stable, biocompatible materials (e.g., PEEK, titanium alloys) while balancing cost-effectiveness. CDMOs often provideDFMEA tools to mitigate risks.
6. Material Innovation Trends in High-End Medical Devices
Current trends emphasizehigh-performance biomaterials:
•Orthopedics: Porous tantalum, ceramic composites for enhanced bone integration.
•Cardiovascular: Biodegradable polymer scaffolds replacing traditional metals.
•Wearables: Flexible circuits and liquid metals for next-gen smart devices.
CDMOs maintain proprietary material libraries and collaborate with research institutions for patented formulations.
7. Selecting a One-Stop CDMO Partner: Key Criteria
Evaluate providers based on six dimensions:
1.Technical Completeness: Full-service capabilities from design to certification.
2.Production Scalability: Cleanroom capacity, equipment redundancy, and rapid response times.
3.Intellectual Property Protection: Robust data encryption and NDA enforcement.
4.Global Regulatory Expertise: Track record of successful CE/FDA approvals.
5.Cost Transparency: Detailed pricing models with no hidden fees.
6.Risk Mitigation: Supply chain redundancies and contingency plans. Recommend audits and pilot projects for validation.
8. Innovative CDMO Solutions Addressing Industry Pain Points
Leading CDMOs offer tailored solutions:
•Expedited Pathways: Combined biocompatibility testing and parallel regulatory submissions, slashing time-to-market by 40%.
•Flexible Manufacturing: Hybrid approaches (3D printing + CNC machining) lowering minimum order quantities.
•Digital Twin Technology: Virtual validation systems reducing physical prototypes. For example, a ventilator component project cut design cycles by 60% and defects to0.12 ppm.




Reviews
There are no reviews yet.